Introduction:
Total body irradiation (TBI)-based conditioning was found to be associated with superior leukemia-free survival when compared to chemo-based conditioning for children with ALL undergoing allogeneic hematopoietic stem cell transplantation (HSCT) in the prospective, international, randomized FORUM trial (Peters et al, JCO 2021). Nevertheless, TBI is considered as the major risk factor for developing secondary malignancies (SM). We and others have previously reported the incidence, risk factors, and outcomes of SM after HSCT in children with ALL (Eichinger et al, Leukemia 2022), but prospective studies of SM after HSCT in children are limited. Therefore, we analyzed the results of 2151 patients enrolled in the prospective BFM 2003 and the FORUM trial from 2003-2022.
Patients and methods:
We included data from all 668 patients (pts) treated in the BFM 2003 trial and from all 1483 patients treated in the FORUM trial with HSCT from HLA-identical family donors (MFD, 26% of patients), matched unrelated donors (MUD, 65%), and either related or unrelated mismatched donors (MMD, 9%).
In the BFM 2003 trial pts >2 years (y) of age years were offered TBI/VP-16 (12 Gy TBI in 6 fractions of 2 Gy each, given as 2 fractions per day for 3 days; VP-16 60 mg/kg, maximum total dose 3600 mg) as conditioning. Only pts with contraindications for TBI received chemo-conditioning, which consisted of i.v. busulfan, cyclophosphamide and etoposide.
In the FORUM trial pts >4y of age were randomized to receive either TBI- or chemo-based conditioning regimens; pts <4y were allocated to the chemo-conditioning arm consisting of fludarabine/thiotepa and either busulfan or treosulfan. Details on the transplant procedure have been previously described (C. Peters, et al. JCO 2015 /2021).
Results:
Centres from 31 countries on 5 continents contributed to the two studies. The median age was 9.2 (range 0.5-23) y. The majority were male (63%, 1356/2151) and >2 y of age at HSCT (94%, n=2024). TBI-based conditioning was applied in 64% (n=1429), while 34% (n=722) of pts received chemo-conditioning. TBI/VP16 was given in 27% (43/159) of age group 2-4y, and in 76% (1269/1672) of age group >4y.
With a median follow-up of 3.29 (range 0.1-16.4) y, a total of 46 SM (33 in BFM 2003, 13 in FORUM) were diagnosed at a median of 5.12 (range 0.4-13.4) y post HSCT, all but one in the TBI group. Four pts experienced ALL relapse prior to the occurrence of the SM leaving 42/2151 cases for further analysis.
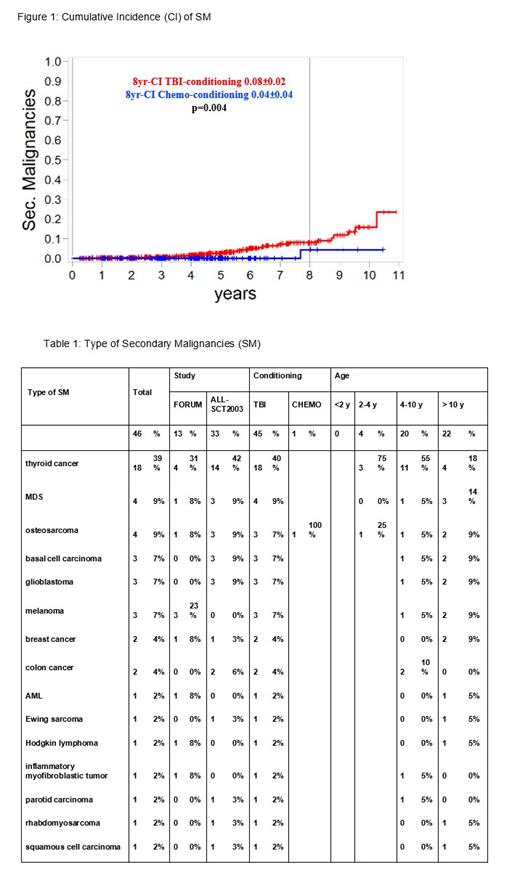
The 5-, 8-, and 10-y cumulative incidence (CI) of SM, with relapse and non-relapse mortality being competing events, were 0.02 (±0.01), 0.06 (±0.01), and 0.13 (±0.03), respectively. Details of the SM are described in Table 1.
Regarding age at HSCT, no SM was observed in the very young age group (<2 years) (0/127), 4 SM in the 2-4 years group (2.2%, 4/181), 20 SM in the 4-10 years group (2.3%, 20/867), and 22 SM in the >10 years group (2.25%, 22/976) (p=n.s.).
Six patients developed an additional SM: these were MDS (n=2), and glioblastoma, breast cancer, basal cell carcinoma, and squamous cell carcinoma (one each). The first SM occurred at a median of 5.12 (range 0.4-13.4) y, and the additional SM at 10.7 (range 4.3-12.1) y after HSCT.
The probability of OS after SM diagnosis was 0.65 (±0.008) at 5y and 0.32 (±0.17) at 10y. Causes of death were SM in 12/14 cases and 2/14 ALL relapse-associated. All 3 glioblastoma-pts died within 8 months after diagnosis. 17/18 pts (94%) with thyroid cancer were alive at last follow-up (0-10 y after SM diagnosis).
As shown in Figure 1, the 8-y CI of SM differed significantly between TBI- and chemo-conditioning (0.08[±0.02] versus 0.04[±0.04], p=0.004) in both age groups (2-4 y and >4 y).
No significant correlation was observed with patient characteristics such as age, gender and ALL remission status.
In addition, we did not identify other statistically significant transplant-specific risk factors including donor type, stem cell source, grade II-IV and III-IV aGVHD and cGVHD.
Conclusions
Updated data from two prospective studies using uniform regimens confirmed TBI-based conditioning as a risk factor for SM in pediatric ALL. Further analysis is ongoing regarding the association with immune phenotype of ALL and details of genetic risk factors (e.g. TP53 mutations). Rigorous long-term FU of TBI patients is mandatory.
Disclosures
Lawitschka:novartis: Consultancy. Dalle:blue bird bio, Incyte, Jazz Pharm. Medac, Sanofi, Vertex: Membership on an entity's Board of Directors or advisory committees; Novartis, Orchard: Membership on an entity's Board of Directors or advisory committees, Other: speaker (symposia). Büchner:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Díaz De Heredia:Novartis: Membership on an entity's Board of Directors or advisory committees, Other: travel expenses, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Other: travel expenses; Prothya Biosolutions: Research Funding; Biotest: Consultancy. Meisel:Miltenyi Biotech: Research Funding; Vertex: Consultancy, Research Funding, Speakers Bureau; CRISPR Therapeutics: Consultancy, Research Funding, Speakers Bureau; Bluebird Bio: Consultancy, Speakers Bureau; CELGENE BMS: Consultancy, Research Funding, Speakers Bureau; medac: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau; Gilead/KITE: Research Funding. Ansari:Jazz Pharmaceutical: Other: traveling grant and presentation inside the company on HSCT; NovoNordisk: Other: traveling grant. Balduzzi:Novartis, Amgen, Medac, Neovii: Speakers Bureau. Peters:Riemser, Medac: Honoraria; AOP Orphan Drugs, Jazz, Neovii: Other: Meeting/Travel grant; Novartis: Consultancy; Novartis, AMGEN: Membership on an entity's Board of Directors or advisory committees; AMGEN, Neovii, Jazz: Research Funding. Bader:Novartis: Consultancy, Research Funding; Neovii: Research Funding; BMS: Research Funding; Medac: Consultancy, Patents & Royalties: medac, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal